Clients
AbtBioConsult has supported international clients from several continents, often on long term or recurrent projects. These include small and mid-size Biotech, large and global Biopharma, Investment banks and Foundations, as well as Service providers in the field of biotherapeutics.

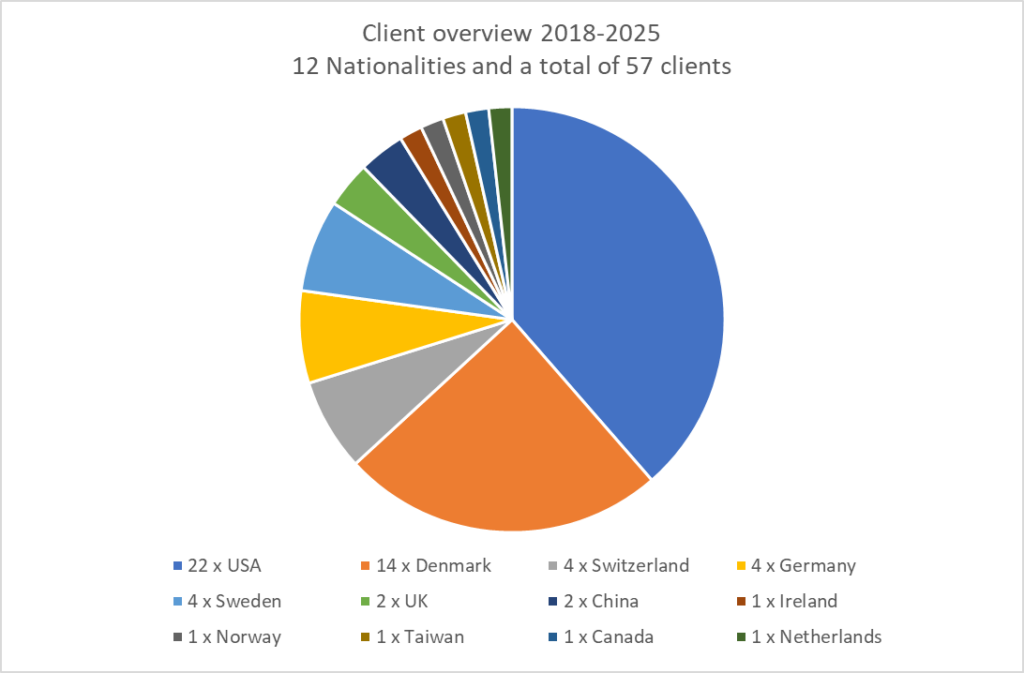
AbtBioConsult clients
Client examples










Testimonials
“Working with Anne has been a pleasure. She is always knowledgeable, efficient and practical. Having a consultant with her level of expertise was greatly beneficial to our project.” CEO, Notogen
“We have had the pleasure of working with Anne on a number of diligence projects over the years. Her knowledge and experience have been invaluable in supporting our evaluation of potential investments. Importantly, our target company management teams have been impressed by Anne’s in-depth sector knowledge, experience and approach and this reflects well on us as a potential investor.” GHO Capital Partners
“Anne has been instrumental in guiding our CDMO selection for IND-enabling activities, providing deep expertise across the range of early CMC processes.” ManifoldBio
“Anne has been our external expert advisor for the development of our novel antibody platform. Her technical expertise and comprehensive understanding of the field have been instrumental in advancing this technology, especially her guidance on process development and regulatory requirements.” CSO, Memo Therapeutics
“MinervaX has engaged AbtBioConsult for various projects, including cell line development and documentation, as well as general consulting within Chemistry, Manufacturing, and Controls (CMC). Throughout our collaboration, AbtBioConsult, under Anne’s leadership, has consistently delivered high-quality, actionable insights. Anne has been a pleasure to work with, offering timely, relevant, and highly practical advice that has greatly supported our initiatives.” CTO, MinervaX
“As an instrument provider, navigating regulatory requirements can be complex and daunting. Anne brings a comprehensive yet pragmatic approach, helping us clearly differentiate between essential ‘must-have’ features and ‘nice-to-have’ enhancements for our products. Her guidance has been invaluable in ensuring we meet compliance without overcomplicating our offerings.” CEO, Seed Biosciences
“Thank you, Anne, for your invaluable insights into our upstream process development. We greatly appreciate your extensive knowledge and emphasis on thorough documentation, along with your ability to describe various process aspects clearly. Your contributions have significantly enhanced our ability to make decisions.” CMC & Device Lead, LEO Pharma
“For many years, our company has received a very professional advice from Anne in connection with the company’s development of new services in the field of protein development and manufacturing. Anne is amongst the most competent advisors within the mammalian cell upstream area that we know of and we highly value the stringent way she works as a consultant.” CEO, Bioneer
“Anne has supported us in our CDMO selection process, including request for proposal and review of proposal/scope of work. She has significant experience within the field and has a strong background within the different scientific disciplines. Her participation has been important in our discussions with the different CDMOs and in the final selection process.” Biopharma company